Assistant Professor
Email: arobbins@uttyler.edu
Building: RBN 3006
Department: Mechanical Engineering
Popular Searches

Assistant Professor
Email: arobbins@uttyler.edu
Building: RBN 3006
Department: Mechanical Engineering
Dr. Robbins’ research focuses on orthopedic and whole-body biomechanics, tissue biomechanics,
and medical device design and entrepreneurship. He is currently engaged in projects
evaluating orthopedic devices used in horses, developing whole body biomechanical
models of sheep for pre-clinical orthopedic studies, developing novel mechanical testing
apparatuses for biological tissues and tissue engineered grafts, and mechanically
evaluating novel 3d printed structures for biomedical applications.
Unlocking the secrets of human and animal movement. Our biomechanics research explores the intricate workings of bodies to understand and detect disease and injury, and to improve performance. Dive in to discover how we’re pushing the boundaries of human potential:
 Figure 1: A sheep model of the genetic disorder hypophosphatasia (HPP).
Figure 1: A sheep model of the genetic disorder hypophosphatasia (HPP).
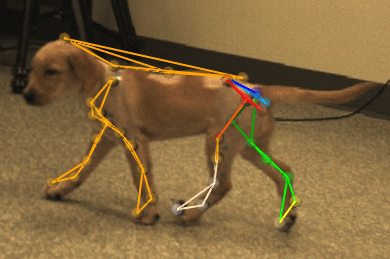 Figure 2: A dog model of muscular dystrophy.
Figure 2: A dog model of muscular dystrophy.
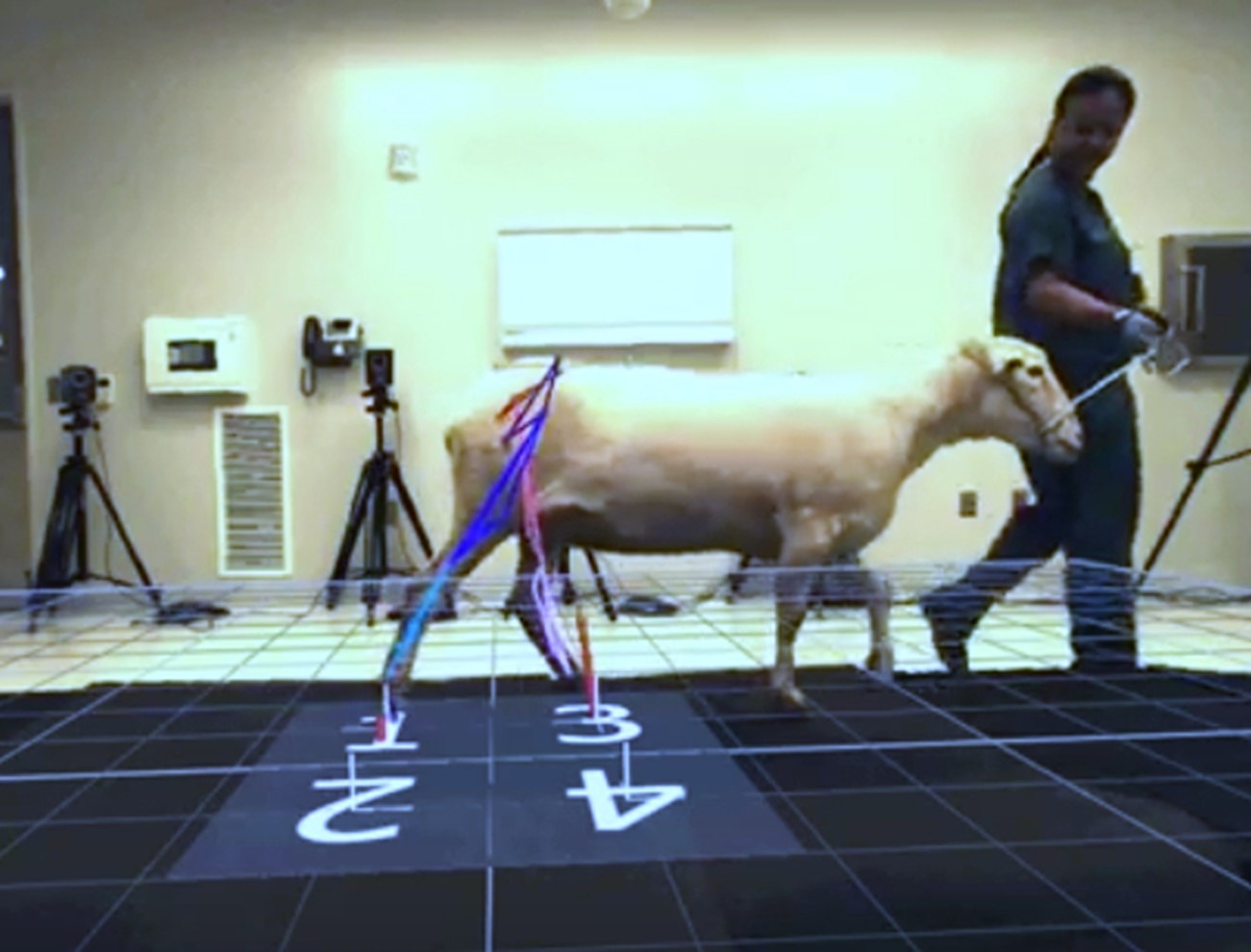 Figure 3: This sheep has an implanted novel fracture fixation device.
Figure 3: This sheep has an implanted novel fracture fixation device.
Large animals such as horses, dogs, pigs, and sheep are often used to study musculoskeletal diseases, orthopedic procedures, and the biomechanical impacts of these conditions on gait and other biomechanical parameters. These studies are often intended to be translational in the sense that they are expected to provide insight into related conditions in humans, or predict performance of devices or therapies when translated to humans. However, there is a large capability gap in biomechanics between large animal studies and human studies; decades of development has resulted in sophisticated inverse dynamic models and related tools for human biomechanics that are not available for large animals. This relegates biomechanical endpoints in large animal studies to external measurements (such as ground reaction force), or simple and less than accurate kinematic measures (like joint angles) estimated from 2D video, or from 3D surface markers. In current practice, internal biomechanical parameters are not easily determinable (e.g. joint reaction forces and torques, muscle activation). The goal of this work is to develop and validate an inverse dynamic model for sheep that can then be employed to study biomechanical outcomes in animal models of human disease. We believe this will provide a useful tool in the study of musculoskeletal diseases and conditions, and their treatments.
Biomechanical analyses such as gait analysis have found research and commercial utility in a variety of contexts in livestock. A particularly salient example is the assessment of lameness in cows/steers; each lame cow can cost a producer several hundred dollars of lost milk production, reduced fertility, increased risk of culling, etc. Lameness prevalence in commercial herds remains high, at over 20%. Similarly, lameness in sheep results in reduced fertility and lower growth, and is largely caused by infectious disease, and lameness in pigs may have a prevalence from 5-20% and cost tens or hundreds of dollars per animal. Beyond lameness, biomechanical measurements may also be sensitive to neurological diseases that can cause gait and other motion abnormalities, including bovine spongiform encephalopathy, scrapie, and encephalitis, and other disease states that can affect motion such as ketosis and hypocalcemia. Ultimately, biomechanical analysis has the potential to identify lameness and other conditions quickly and reliably, reducing the costs of lost productivity and the spread of communicable diseases.
Our research delves into the intricacies of human motion, leveraging cutting-edge technology to understand how our bodies move and perform. In collaboration with the Biomechanical Environments Laboratories at Texas A&M we utilize motion capture systems, which employ specialized cameras and markers to precisely track and analyze movement in three dimensions. This allows us to study everything from the subtle nuances of an athlete’s gait to the complex coordination involved in everyday tasks. In addition to motion capture, we employ other advanced techniques such as electromyography (EMG) to measure muscle activity, and force plates to quantify ground reaction forces. By combining these methods, we gain a comprehensive understanding of the biomechanical factors that influence human movement, enabling us to develop innovative solutions for improving performance, preventing injury, and enhancing rehabilitation.
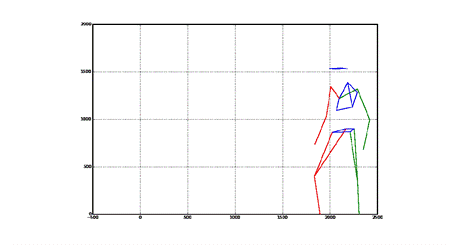 Figure 4: Motion capture results of an MMA fighter performing acrobatic motions.
Figure 4: Motion capture results of an MMA fighter performing acrobatic motions.
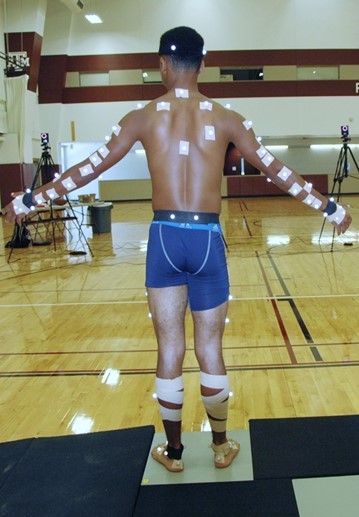 Figure 5: A high school quarterback participating in a motion capture study
Figure 5: A high school quarterback participating in a motion capture study

We produce novel testing methods and apparatus to answer complex biomechanical questions in applications such as orthopedic surgery, tissue engineering, and mechanics of materials. Pictured above is a testing mockup for a cantilevered bending test for a canine metatarsal. This experiment was designed to evaluate two different fracture fixation methods.
View a video of a screw pull-out test on an equine (horse) metatarsal. This 5.5mm buttress threaded screw implanted mid-shaft into the bone required over 1,500 pounds of force to cause failure of the bone:
This experiment shows a physiological compressive bending experiment on hocks taken from horse cadavers. This study is comparing the strength of different methods for fusing this joint (arthrodesis) which can save a horse’s life if they are injured.
Below you can see a similar screw insertion test on a bone screw fastener implanted into an equine metatarsal.
 Figure 7: Screw insertion testing on a equine metatarsal bone.
Figure 7: Screw insertion testing on a equine metatarsal bone.